Water consists of hydrogen and oxygen. The process of separating these two elements using electrical force is called electrolysis. If electricity is required to separate the elements’ molecules, can we produce electricity if we find a way to bond them? The answer is yes! And the device that can do it with is called a fuel battery. Shall we try making one?







Materials/Tools you’ll need
 1 x 4-cell battery box (C- or D-size)
1 x 4-cell battery box (C- or D-size)
 1 x motor for solar batteries with propeller
1 x motor for solar batteries with propeller
 3 x alligator-clip wires
3 x alligator-clip wires
 2 x carbon rods
2 x carbon rods
 1 x spoon additive-free salt
1 x spoon additive-free salt
 2 x metal clips
2 x metal clips
 1 x spoon
1 x spoon
 1 x halved purple cabbage
1 x halved purple cabbage
- 1 x beaker (about 500 ml capacity)
- 1 x tall glass or beaker (about 200 ml capacity)
- Hot water
- Aluminum foil

Tear the purple cabbage leaves into pieces and put them in a beaker (500 ml) of hot water. Wait until the water turns purple, then transfer the purple liquid to a tall glass or beaker (200 ml). This is our purple cabbage pigment solution.

This type of pigment solution is called anthocyanin. Its color changes to pinky red when the pH is acidic, and a bluish yellow-green when the pH is alkaline.

Add a little more water to the pigment solution to make its color lighter (to be easier to see). Then throw in 8–10 grams of salt per 500 ml of water and stir well.

We could use regular table salt, but it usually includes additives such as anti-caking agents that may change the color of our solution, so it’s best to stick with additive-free plain salt.

Dip your two carbon rods into the solution and fix them to the rim of the cup with metal clips.

The more of the carbon rod you can immerse in the liquid, the smoother your experiment will be. Fill your glass with as much solution as you can, so the rods are covered enough.

Attach an alligator-clip wire onto the top of each carbon rod. Attach the other end of the alligator wires to the terminals on your battery box.

If it’s difficult to get the clips to stay attached to the carbon rods, try wrapping a 1 cm-wide length of aluminum foil around the tops so the alligator-clips can grasp them securely.

After a while, you’ll notice the solution bubbling around the carbon rods. This indicates that electrolysis is beginning to take place.

The battery’s positive terminal will be acidic, and the negative alkaline. You can tell which is which by the color of the bubbling pigment around the rods, which corresponds to its polarity: pinky red for positive, and bluish yellow-green for negative.

Do not directly connect the alligator-clip wire to the other one, or it will become dangerous and generate heat. Conduct the experiment in a well-ventilated place, as a slight amount of toxic chlorine gas is generated and may cause some discomfort if inhaled.

After 3–5 minutes, disconnect the battery-box from the wires and replace it with the motor with propeller, clipping the wires onto the motor terminals. If the propeller spins, congratulations, you have succeeded in the experiment! You transformed a cup of salty cabbage-water into an electricity-generating device!
If your propeller didn’t spin…
- Check the wiring! If no bubbles start to form around the rods in Step 5, check if the wires are properly connected, and the batteries in the box are installed in the correct orientation.
- Check the concentration of the saltwater solution. If it’s too thin, electric current can’t flow smoothly.
![]() How did bubbles form on the carbon rods?
How did bubbles form on the carbon rods?
Firstly, let's examine the case of electricity conduction in water. In the experimental setup shown on the right, connecting carbon rods and batteries initiates the movement of electrons within the salty water, traversing from the positive electrode to the negative electrode, thereby establishing a flow of electric current (①). Water, which consists of hydrogen and oxygen, undergoes a separation of its molecules into hydrogen and oxygen when subjected to electric current (②). Oxygen atoms accumulate on the positive electrode, while hydrogen ions are drawn towards the negative electrode (③). The formation of bubbles results from the release of hydrogen and oxygen molecules, a phenomenon termed water electrolysis. Note that the reaction in this experiment deviates slightly from typical water electrolysis, as we introduced salt to enhance the conductivity of electricity in the water.

![]() How did the propeller spin?
How did the propeller spin?
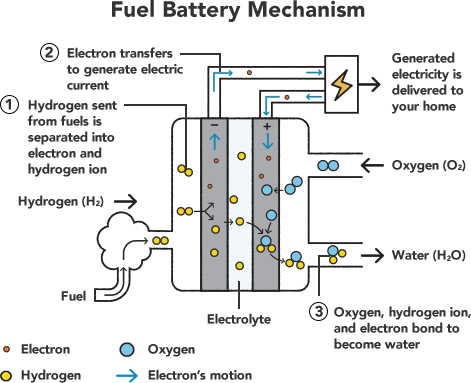
The process of electrolysis separates water into its component elements: hydrogen and oxygen. We achieved this by applying electric force from our dry batteries. However, we can also create electricity by bonding hydrogen and oxygen molecules.
When the battery-box was swapped out for the motor and propeller, this is what happened: first, the hydrogen and oxygen we originally separated recombined back into water (①). At the moment of elemental transition, electrons in the carbon rods moved, creating electrical current (②). Electricity passed through the wire circuit and caused the motor to turn over, spinning the propeller blades (③). Put simply, hydrogen and oxygen—water—worked as a fuel battery.
However, strictly speaking, calling this a fuel battery isn’t 100% accurate because we created a kind of “rechargeable battery phenomenon” by turning water into an electrolyte capable of storing electricity, as well as causing a chemical reaction between hydrogen and oxygen. Yet the basic principle of how a fuel battery actually works is the same.

![]() Battery basics
Battery basics
- Electrolysis is the process of separating water into hydrogen and oxygen atoms through the application of electrical force
- A fuel battery, on the other hand, works by extracting electric energy by encouraging hydrogen and oxygen to react and combine with each other
![]()
- We can make electrolytic solution from materials other than salt. Try experimenting with baking soda (sodium-hydrogen carbonate), vinegar (acetic acid), sports drink (various ions are included), and see which is most effective
- Observe if there is a relationship between the amount of energy generated by the fuel cell and how long the battery box is connected
- We can try the experiment with thick lead (graphite) pencils or sticks of white charcoal instead of carbon rods
What is a fuel battery?
A fuel battery generates electricity by creating and harnessing energy from a chemical reaction between oxygen and hydrogen (or other fuel types). Although there are various kinds of cells that differ by fuel type and structure, one thing they all share is a lack of carbon dioxide emission while discharging, unlike other thermal sources of power, such as the internal combustion engine.
Fuel batteries are therefore the subject of intensive scientific research as a possible alternative to fossil fuels. Fuel-cell cars have already been developed, but other applications might include emergency power-supplies for government offices, public facilities, and hospitals. This is because electricity can be supplied even if there’s a blackout.

- Please keep experiment away from fire.
- Take extra care to avoid touching the wires connected to the battery box. When connecting your circuit, clip the wire onto the battery box last. When pausing the experiment, first disconnect the wires from the battery box. When the battery box is disconnected, do not allow the terminals to contact metal objects. To be safe, remove the batteries from the battery box when not in use.
- This experiment illustrates the principle of how a fuel battery creates electricity by causing a chemical reaction between hydrogen and oxygen. Actual fuel batteries have a different mechanism.